The EtchFree™ evolution:
Introducing the next generation of bonding, inspired by nature
The field of orthodontics has evolved significantly since the earliest braces were applied to the first patients’ teeth. But there remains one aspect of orthodontic technology that is long overdue for innovation: bonding— specifically, the etching process critical to creating a reliable, secure bond.
In 1955, the practice of using phosphoric acid for etching enamel was first introduced in orthodontic practice (Buoncore et. al., 1955), and became widely adopted as the primary method of bonding orthodontic appliances in the 1980s (Sofan et. al., 2017). Since then, orthodontists have regularly relied on etch and self-etch products to prepare the surface of the tooth as the first step of the bonding procedure. Many of these products, however, can have a detrimental effect on the patient’s tooth.
Acid etchant, for example, creates microporosity and a roughened tooth surface. Etching the enamel surface enables primers and resins to seep into the tooth, forming resin tags and creating a mechanical bond. Composite adhesives and orthodontic appliances are then bonded to complete the anchorage. By demineralizing the tooth, these etching processes create morphological changes to the patient’s enamel. This results in enamel loss, allowing the tooth to become vulnerable to white spot lesions throughout the course of orthodontic treatment (Chapman et. al, 2010).
Commonly used etching processes present challenges for the clinician as well as for patients’ enamel health. The etching process is a time-consuming one, requiring clinicians to wait for the etchant product to dissolve highly mineralized enamel. What’s more, etching requires a dry environment to reliably bond orthodontic appliances to teeth. This naturally becomes a challenge, given that the mouth is an inherently wet environment (Phaphe et. al., 2015). Keeping teeth dry enough for technique sensitive etching becomes a stressful and lengthy part of the bonding process.
What if there was a way to remove the etching step entirely, with a system that enables orthodontists to decrease technique sensitivity, work confidently in a wet environment, and preserve the integrity of their patients’ enamel? To develop such a system, Ormco’s Research and Development team turned to nature and the field of biomimicry.
Over the past few decades, material scientists have increasingly embraced the biomimicry field, also known as biomimetics, for innovate solutions to a host of product challenges. Indeed, a wide range of human advances have taken inspiration from designs, processes, and applications found in the natural world. Think Velcro (derived from burrs), or bullet trains (influenced by kingfishers), or ventilation systems (borrowed from termite mounds), or swimsuits (adapted from sharks). All these innovations were inspired by time-tested evolved principles gleaned from the observable world.
Could we not apply nature’s inspiration to orthodontic bonding? In its simplest mechanical terms, orthodontic bonding is a process by which appliances are adhered to highly mineralized surfaces (patient’s teeth).
The challenge of successful bonding lies in the biologically active and highly moisture contaminated oral environment of the patient’s mouth. This environment can interfere with the chemical processes orthodontists commonly use in bonding. Adding to the challenge, clinicians must ensure that the adhesive they use is not only effective but also safely applied. To address this challenge, Ormco R&D turned to biomimicry to explore different mechanisms used by various species to selectively bond or adhere to surfaces. Our research led us to marine mussels.
Marine mussels were of particular interest to us because they exist in physically turbulent aquatic environments that are rife with chemical and biological contaminants— much like the mouth. These creatures have adapted to their moist environment, evolving an exceptional ability to attach themselves to myriad substrates, including highly mineralized rocks. Could human invention find inspiration from their unique adaptations?
To find out, we must first understand how marine mussels achieve this remarkable adhesive quality in such challenging conditions. Their bonding ability involves a combination of chemical and mechanical actions. The marine mussel’s anatomy includes the byssus, a threadlike structure that extends from the mussel stem. This byssus contains 25 to 30 different foot proteins, pre-polymerized collagens, and thread matrix proteins that form a complex bonding network (Lee et. al., 2011).
The byssus proteins contain a high quantity of post-transitionally modified 3,4- dihydroxyphenylalanine (DOPA). A chemical group containing a catecholamine and a phenethylamine, DOPA is found in dopamine and epinephrine. It’s also widely thought to be the critical ingredient that allows mussels to adhere to a variety of substrates (wood, metal, mineral, and more) in wet environments and turbulent conditions. This durable bond, even in challenging circumstances, is further supported by a cross-linked polymer matrix that forms when seawater’s basic pH and oxidizing properties combine to create a catechol oxidation that produces quinone (Pinnataip, et al., 2021).

Figure 2. Exemplary marine mussel attachment (rocks and vessels).
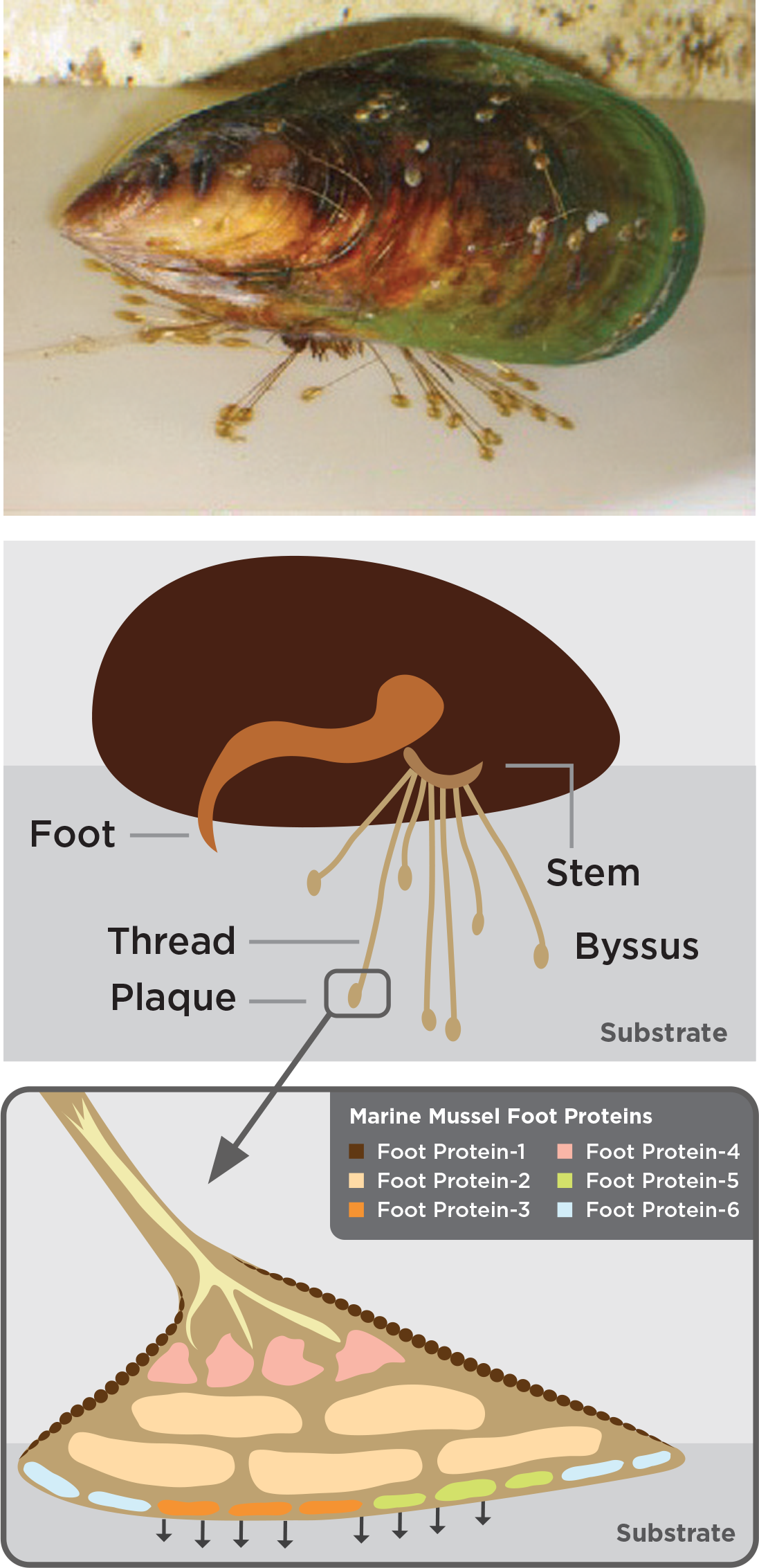
Figure 1. Diagrams of marine mussel attachment to a substrate.
There are striking parallels between the nature of marine mussel bonding to mineralized surfaces in aquatic environments, and the clinical requirements of bonding to mineralized tissues (i.e. tooth enamel) in the oral environment. In other words, the bonding of mussels to rock is analogous to the bonding of brackets to teeth.
Like sea water, saliva is generally neutral to slightly basic pH and saline, and harbors highly mineralized substrates. As such, it only seemed natural to apply the biology and chemistry behind the bonding-proficient marine mussels. The Ormco R&D team synthesized a monomer inspired from marine mussel proteins that enable these animals to adhere so effectively to substrates in wet and challenging environments. This proprietary monomer is used as the foundation for our Ormco EtchFree™ Bonding system, designed to eliminate the time-consuming and often frustrating etching step, preserve the patient’s enamel, and deliver a robust bond in a wet and biologically active environment.
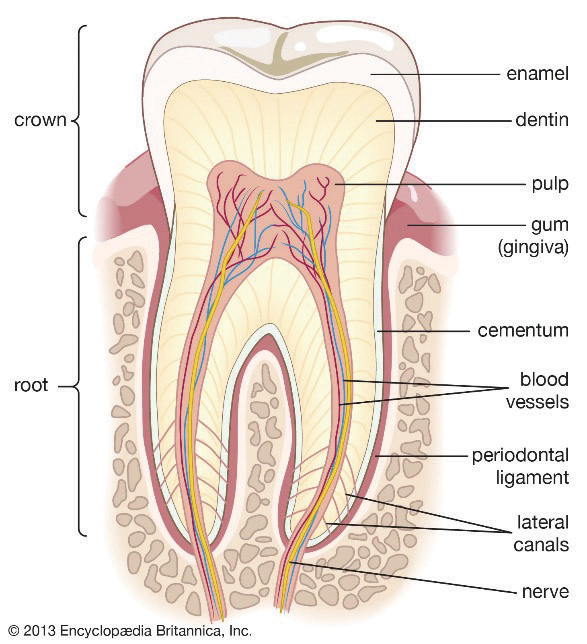
Figure 3. Cross section of a tooth
The next evolution in orthodontic bonding, the Ormco EtchFree Bonding system removes the etching step and thus the need for phosphoric acid as the active ingredient for prepping the tooth’s surface. The system simplifies the boding workflow with a three-step process.
Designed to replace the etchant step, minimizing tooth damage and maximizing efficiency, EtchFree Bonding Primer is applied directly onto the pumiced tooth. Waiting, scrubbing, or typical rinse and dry steps are no longer required, saving clinical teams and patient’s time.
Applied on top of the EtchFree Bonding Primer, the Ortho Solo product enhances bond strength between the tooth and the orthodontic appliance.
In this final step, the EtchFree Adhesive provides a reliable bond with metal brackets, ceramic brackets, or as an aligner attachment. Designed to be compatible with the doctor’s preferred orthodontic prescription, EtchFree Adhesive has the same curing time as other Ormco adhesives and is translucent after curing. Let’s dive into the EtchFree Bonding Primer and EtchFree Adhesive steps of the bonding process in greater detail.
The EtchFree Bonding system delivers bond strengths that last the duration of the orthodontic treatment. For a useful life of three years, bond strengths of orthodontic appliances with any adhesive system should be at least 5.9 mPa (Reynolds et al., 1976). Current adhesive systems have bond strengths greater than 8 mPa to minimize the risk of debonds while not exceeding 13.5 mPa to prevent enamel damage when brackets are debonded (Bayani et al., 2015, Boruziniat et. al., 2015). The EtchFree Bonding system has average bond strengths of 10 mPa or greater with metal brackets, ceramic brackets, and aligner attachments, ensuring a durable bond with minimal risk of fracturing enamel during debond appointments.
While typical etchants demineralize the enamel surface, EtchFree Bonding Primer uses a proprietary monomer inspired by proteins found in marine mussels to chemically prepare the tooth surface. This monomer cross-links with itself and other polymers in the system to form a matrix of co-polymers with specific adhesion properties (Lee et al., 2011).
Functional groups in the EtchFree Bonding Primer only react once the primer has been applied to the tooth surface. Once applied onto enamel, the unreacted functional groups ionically bond to the calcium ions found in the hydroxyapatite that comprise 96% of the enamel by weight.
Hydrogen bonding also occurs between the cross-linked polymers and the hydroxyapatite. This combination of hydrogen and ionic bonding ensures the tooth surface is ready for Ortho Solo and EtchFree Adhesive applications. The cross-linked primer layer is moisture tolerant, preventing water from inhibiting the chemical bond between enamel and the EtchFree Bonding Primer.
EtchFree Bonding Primer is additionally designed to replace the etchant step without damaging the tooth. Etchant contains phosphoric acid and leaves a frosty, roughened surface after application. In contrast, EtchFree Bonding Primer contains no phosphoric acid, and results in a glossy finish on the enamel surface after a primer coat is applied. Indeed, SEM images show that EtchFree Bonding Primer is less aggressive than selfetch products containing phosphoric acid. This helps preserve the natural surface of the tooth and presents a superior alternative over current etch and self-etch products when it comes to enamel health.
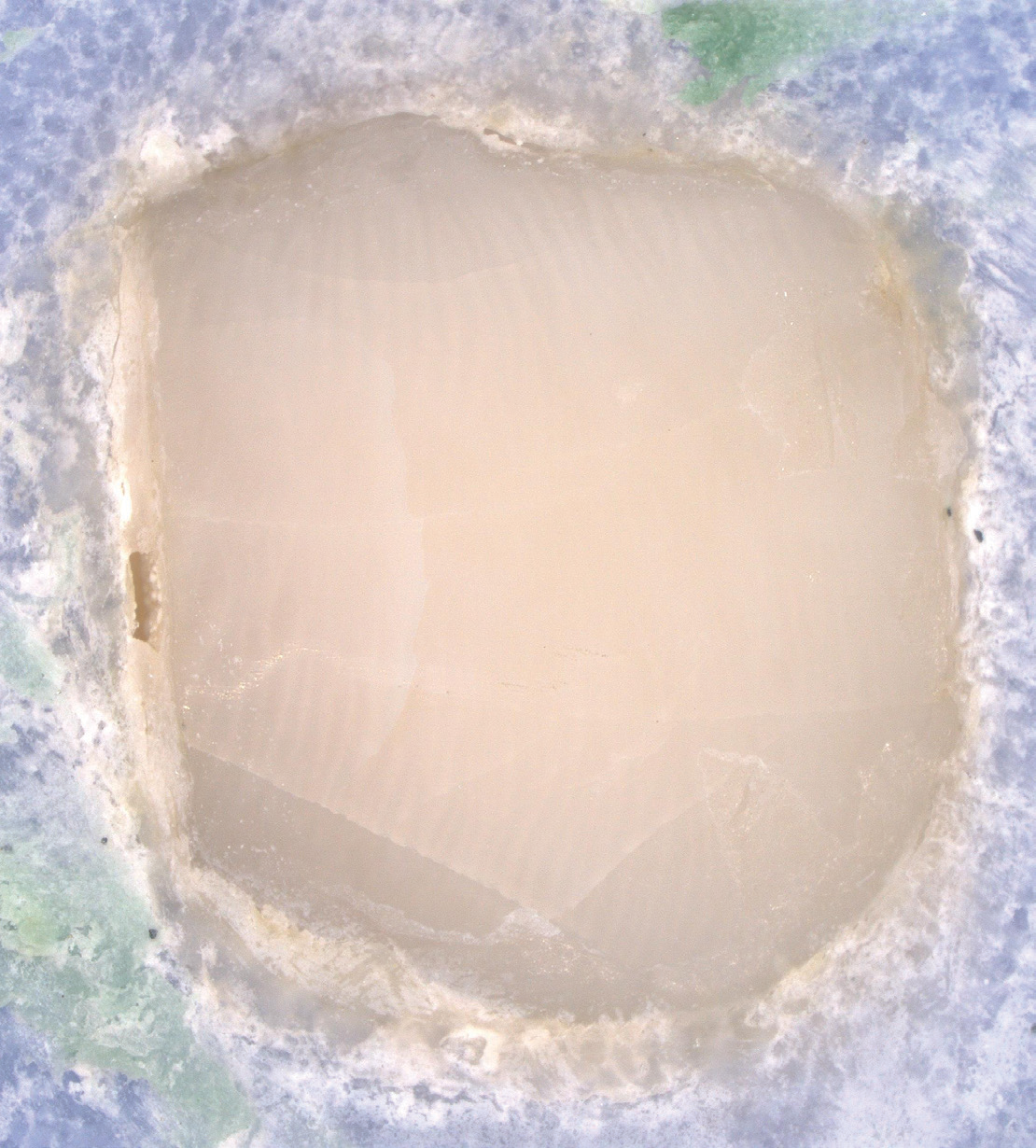
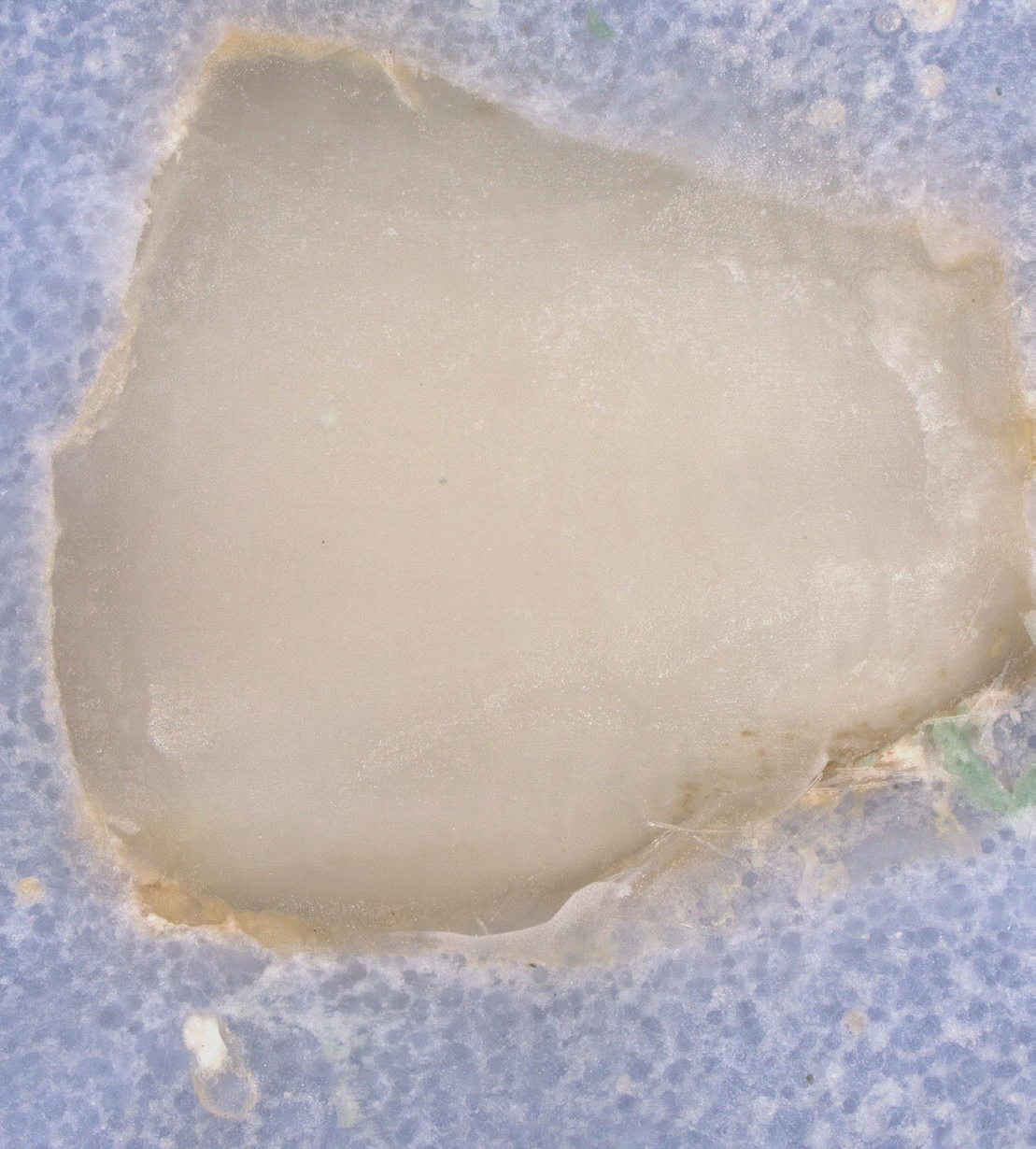
Figure 4: Images of teeth after application of EtchFree Bonding Primer (left) and 37% phosphoric acid etching solution (right). EtchFreeBonding Primer leaves a glossy finish on the surface of the tooth, indicating minimal (if any) surface roughening when compared toetching solution.
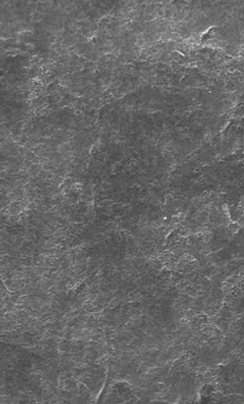

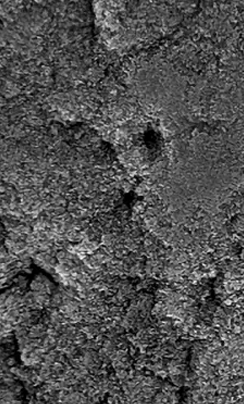

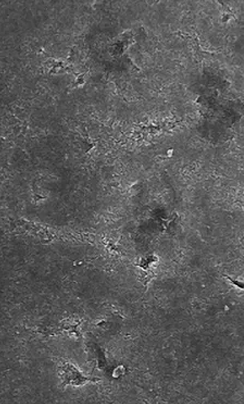
Images shown at 5,000x magnifi cation. 5 Kerr Self-Etch is a trademark of Kerr Corporation. 6 3M™ Transbond™ Plus Self Etching Primer is a trademark of Solventum.
Figure 5: SEM images of teeth after application of EtchFree Bonding Primer (far right) and competitor products (middle three) whencompared to virgin enamel. EtchFree Bonding Primer creates less microporosities compared to etch and self-etch competitor products,indicating more intact enamel.
A new composite formulation from Ormco, EtchFree Adhesive is translucent while delivering a familiar feel and handling. With an easily manipulated consistency, EtchFree Adhesive integrates properly into the bracket pad and can be molded to fi t the shape of an aligner attachment. With a curing time on par with other Ormco adhesives used in conjunction with metal and ceramic brackets as well as aligner attachments, EtchFree Adhesive delivers a bonding strength that spans the life of the orthodontic treatment.
Sharing EtchFree Bonding Primer’s patented technology, EtchFree Adhesive performs in moist environments. When paired with EtchFree Bonding Primer, EtchFree Adhesive allows clinicians to bond in wet fi elds while maintaining the experience expected from a world-class orthodontic adhesive.
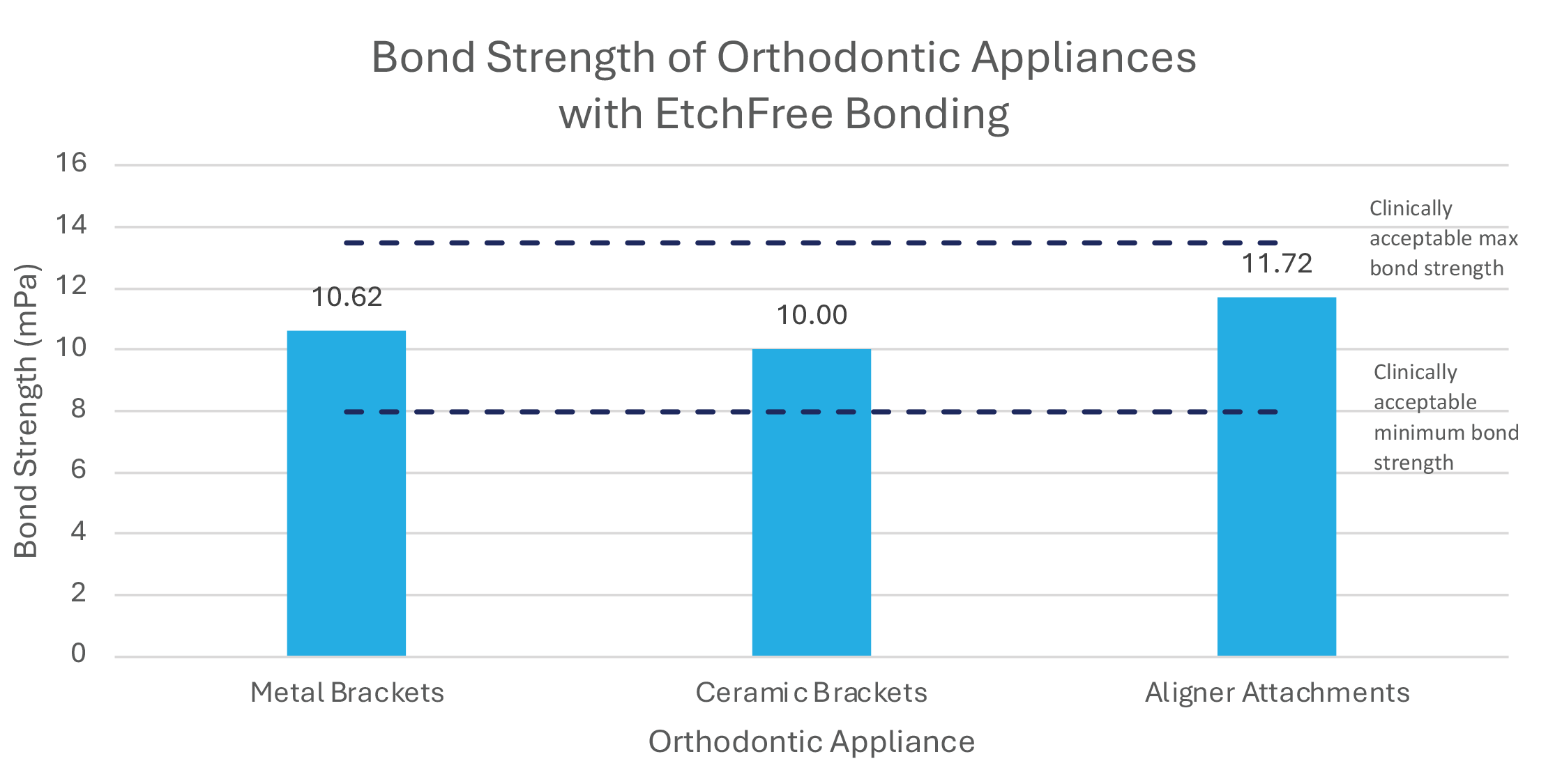
Graph 1: Bond strength performance of EtchFree Bonding system with each orthodontic appliance (sample size n=36 for each group).

Aligner Attachments
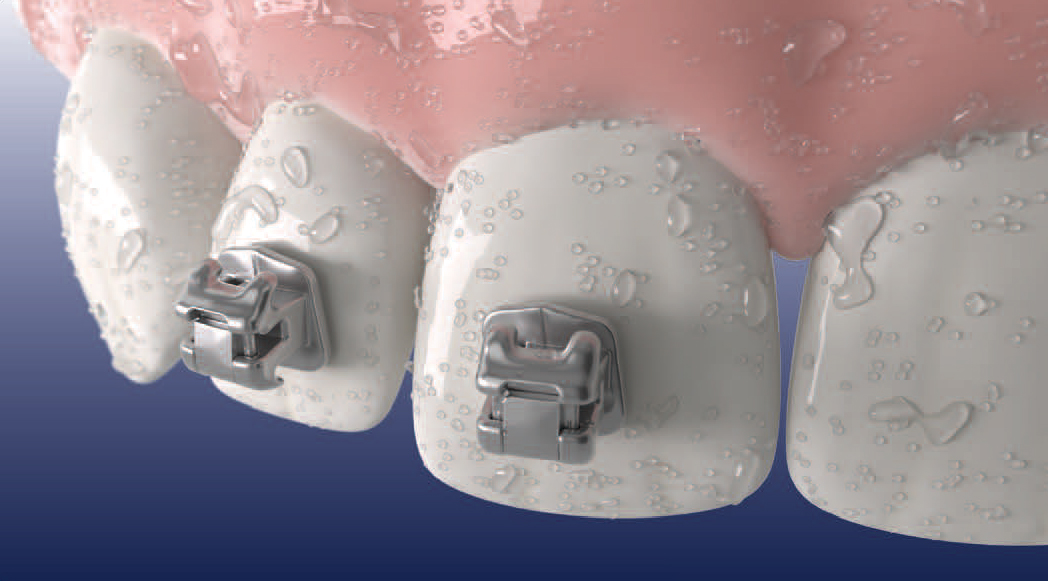
Metal or Ceramic Brackets
One of the most significant challenges in the bonding process stems from the wet field and moisture contamination that leads to debonds. Products that employ a traditional etch-and rinse method simply don’t perform when moisture compromises the bonding system (Prasad, et al., 2014).
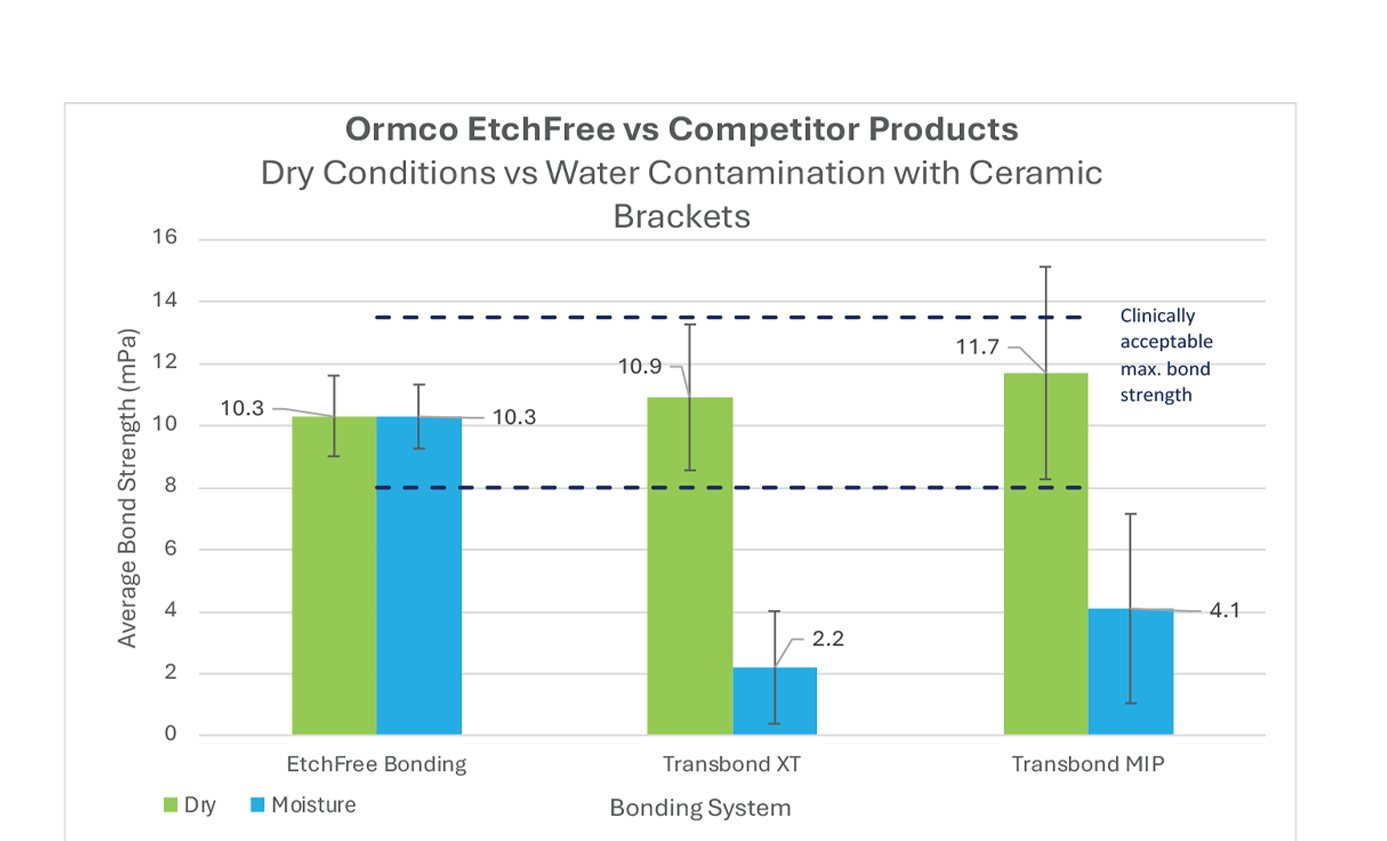
Graph 2: Bond strength performance of EtchFree Bonding system, Transbond XT, and Transbond MIP bonded to ceramic brackets in dry and wet conditions (sample size n=36 for each group). Wet conditions are defi ned as moisture introduced at every step of the bonding process.
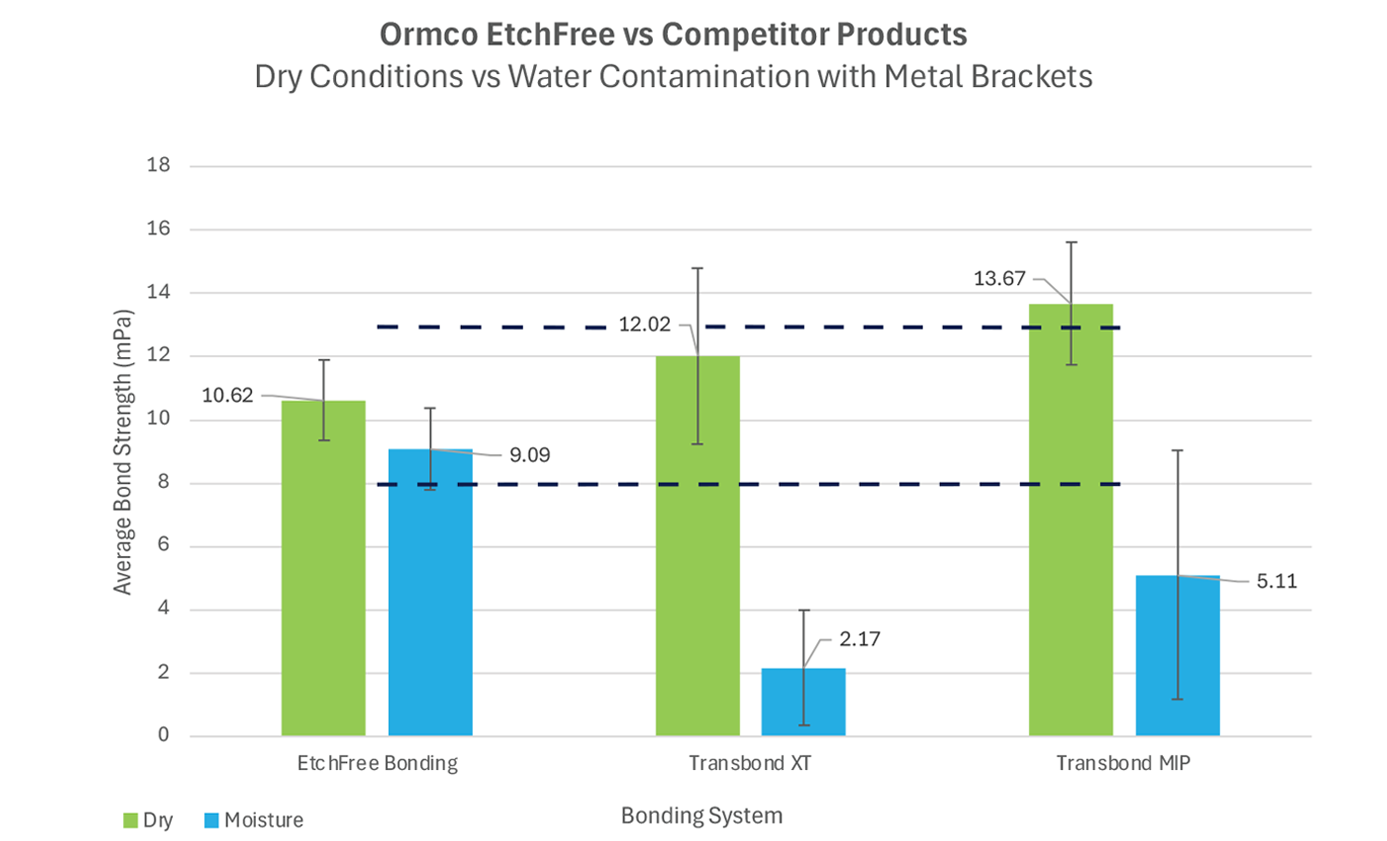
Graph 3: Bond strength performance of EtchFree Bonding system, Transbond XT, and Transbond MIP bonded to metal brackets in dry and wet conditions (sample size n=36 for each group). Wet conditions are defi ned as moisture introduced at every step of the bonding process.
While tests indicate that competing products can have a higher bond strength average in dry conditions, the statistical variance has a greater range than EtchFree Bonding System and reaches the threshold for possible enamel damage. In dry and wet conditions, EtchFree Bonding System maintains a consistent bond strength that meets clinically acceptable thresholds for orthodontic treatment without reaching the threshold of potential enamel damage during debond appointments.
Along with better bond strength in wet and dry conditions, the EtchFree Bonding system delivers workflow improvement by reducing the time and technique sensitivity required to bond orthodontic appliances. Doctors no longer need to wait, scrub, rinse, or dry for the EtchFree Bonding Primer to chemically bond to enamel, reducing chair time for patients as well as prep time for staff.
For even more efficiency gains, the Ortho Solo sealant can be applied immediately after the EtchFree Bonding Primer has been applied. Since neither the EtchFree Bonding Primer nor Ortho Solo need to be rinsed or cured, both products can be applied in as little as 20 seconds per tooth. This improved workflow translates into overall chairtime savings, and was confirmed in Ormco clinical trials. In a split mouth study, 85 patients were bonded with metal brackets using etchant-based bonding systems as a control group and EtchFree Bonding to compare overall bonding procedure time. The study concluded that EtchFree bondings took 20% less time to complete than etchant-based systems, equating to a projected time savings of over 5 minutes for a full bonding.
Emergency or incidental debonds are also a major factor and metric for an orthodontic office’s overall productivity and health. Clinical trials observed a reduced debond rate compared to industry averages. Of 57 patients who completed the 6-week study, incidental debonds (excluding technique failures) occurred at a rate of 2.1% (Ormco data on file) versus an industry average of 4.7% (O’Brien KD et al, 1995).
Combined, the more efficient bonding procedure and reduced incidental debonds translate to significant improvements to office efficiency and productivity.
A Split mouth study comparing etching and EtchFree systems was conducted on 85 patients with metal brackets.

94% of Clinicians and staff members felt “very noticeable” or “somewhat noticeable” time savings with EtchFree Bonding.
Traditional Etch, Prime, Bond Procedure – 11 Steps










Ormco EtchFree Bonding – 9 Steps








Figure 6: Average time savings of EtchFree Bonding system compared to the conventional etch and rinse method used in Ormco clinicaltrials when bonding metal brackets. (above). There is no need to rinse and dry the tooth after EtchFree Bonding Primer has been appliedonto the tooth (below).

Dental and orthodontic bonding techniques and technology have been long overdue for innovation. The latest advancements in bonding came with the 9th Generation adhesives (Sofan et al., 2017). Often referred to as universal adhesives, 9th Generation adhesives incorporate a number of attractive features, including:
While generally capable of successful bonding when used in a self-etch process, academic and clinical data indicate that 9th Generation products deliver better bond strength and long-term reliability when applied after etching and rinsing (Bhattacharjee et al., 2021). For this reason, many clinicians choose to continue using earlier generation products that require separate etching and priming steps in order to achieve clinical efficacy (Asarsa et al., 2025). This means that many clinicians are not benefiting from the workflow efficiencies promised by 9th Generation products.
It’s true that many adhesive formulations used in 9th Generation create ionic bonds to hydroxyapatite through adhesive polymer complexes (as does the EtchFree Bonding system). However, the ionic bonds in these 9th Generation products do not result in high enough bond strength to meet clinical requirements due to chemical limitations or polymer complexes’ sensitivity to the contamination-prone oral environment. Because of this, it is still necessary to create microporosities in the enamel or most reflective surfaces to facilitate a mechanically retentive bond with cured resin adhesives (Sofan et al., 2017).
So despite the generational advancements made over the past several decades, the clinically challenging and technique-sensitive etching step remains key to creating a strong enough bond to support most restorative or orthodontic clinical scenarios. Until now.
The EtchFree Bonding system requires no acid etching mechanism to create clinically successful bonds in orthodontic cases. This puts the system in stark contrast to the popular orthodontic bonding methods that preceded it. That’s because the co-polymers in the EtchFree Bonding Primer bond instantly to the calcium ions in hydroxyapatite while remaining impervious to moisture. These ionically bonded polymer groups remain active and available for following resin adhesive steps, creating the bond strengths needed for successful treatments.
Though still in its first iteration, the EtchFree Bonding system opens exciting possibilities for future development pathways and applications. With its unique formulation, mode of action, and current capabilities, the EtchFree Bonding system is the beginning of a potential paradigm shift in dental adhesive technologies. As such, we believe EtchFree represents the first product of the 10th Generation of adhesives.
As it stands, EtchFree has been shown to significantly reduce procedure time, improve patient experience, score high satisfaction by clinical staff, and deliver overall improvements to clinical outcomes in orthodontic applications. EtchFree Bonding provides a chairside solution, ready today, for orthodontists seeking to optimize workflow and minimize risks on bonding day.
References
Buonocore MG. (1955). A Simple Method of Increasing the Adhesion of Acrylic Filling Materials to Enamel Surfaces. Journal of Dental Research, 34(6):849-853.
Sofan, E., Sofan, A., Palaia, G., Tenore, G., Romeo, U., & Migliau, G. (2017). Classification review of dental adhesive systems: from the IV generation to the universal type. Annali di stomatologia, 8(1), 1–17.
Chapman, Joshua A et al. (2010). “Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances.” American journalof orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board ofOrthodontics vol. 138,2: 188-94.
Phaphe, S., Ganiger, C., Ahammed, Y., & Mane, P. (2015). Invitro Study of the Effect of Different Samples of Water Used for Washing the Etchant on Bracket Bond Strength. Journal of clinical and diagnostic research : JCDR, 9(10), ZC53–ZC55.
Lee, B. P., Messersmith, P. B., Israelachvili, J. N., & Waite, J. H. (2011). Mussel-Inspired Adhesives and Coatings. Annual review of materials research, 41, 99–132.
Pinnataip, R., & Lee, B. P. (2021). Oxidation Chemistry of Catechol Utilized in Designing Stimuli-Responsive Adhesives and Antipathogenic Biomaterials. ACS omega, 6(8), 5113–5118.
Reynolds, I. R., and J. A. Von Fraunhofer. (1976). “Direct bonding of orthodontic attachments to teeth: the relation of adhesive bond strength to gauze mesh size.” British journal of orthodontics 3.2: 91-95.
Bayani, S., Ghassemi, A., Manafi, S., & Delavarian, M. (2015). Shear bond strength of orthodontic color-change adhesives with different light-curing times. Dental research journal, 12(3), 265–270.
Boruziniat, A., Khazaei, Y., Motaghi, S., & Moghaddas, M. (2015). Evaluation of bond strength of orthodontic brackets without enamel etching. Journal of clinical and experimental dentistry, 7(4), e519–e523.
Prasad, M., Mohamed, S., Nayak, K., Shetty, S. K., & Talapaneni, A. K. (2014). Effect of moisture, saliva, and blood contamination on the shear bond strength of brackets bonded with a conventional bonding system and self etched bonding system. Journal of natural science, biology, and medicine, 5(1), 123–129.
Bhattacharjee, D., Sharma, K., Sahu, R., Neha, K., Kumari, A., & Rai, A. (2021). Comparative Evaluation of Shear Bond Strength of Brackets Bonded with self Etch Primer/ Adhesive and Conventional Etch/Primer and Adhesive System. Journal of pharmacy & bioallied sciences, 13(Suppl 2), S1168–S1173.
Asarsa, S. K., Mehta, M., Tripathi, M., Johnson, P., Shilpi, M., & Vengala, S. (2025). Comparative Analysis of Shear Bond Strength between Orthodontic Brackets Adhered Using Self-Etching Adhesives and Conventional Adhesives. Journal of pharmacy & bioallied sciences, 17(Suppl 2), S1508–S1510.
O’Brien, K. D., Read, M. J., Sandison, R. J., & Roberts, C. T. (1989). A visible light-activated direct-bonding material: an in vivo comparative study. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics, 95(4), 348–351.
Image references
Qin, Z., Buehler, M. (2013). Impact tolerance in mussel thread networks by heterogeneous material distribution. Nat Commun 4, 2187.
Penn State. (2022, March 25). Invasive Species. Psu.edu. https://sites.psu.edu/krehelycivicissues/2022/03/25/invasivespecies/ Britannica. (2019). Tooth | anatomy. In Encyclopedia Britannica. https://www.britannica.com/science/toothanatomy
All other images are courtesy of Ormco, 2025
MKT-26-0080